PCl3 is a toxic liquid with an unpleasant smell. In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom.

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
What is the molecular shape of BF_3.
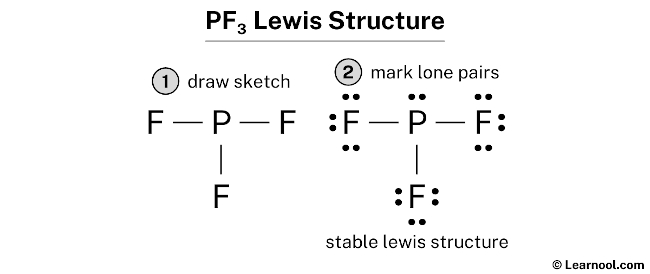
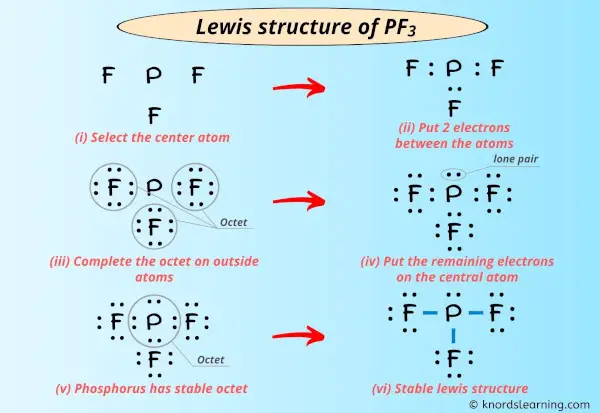
. Weve got the study and writing resources you need for your assignments. Lets do the PF3 Lewis structure. 26 total valence electrons.
SbH3 draw structure. An electron-pair acceptor Sulfur hexafluoride has 6 regions of electron density around the central sulfur atom 6 bonds no lone pairs Drawing lewis structures worksheet with answers Arkansas State University Department of Chemistry and Physics Worksheets Lewis Dot Structures For each of the following draw the Lewis Dot Structure give. Draw the Lewis dot structure for PF3.
So this is our following Luis structure and it has a try. And then we fell in our offense. Start your trial now.
The melting point and boiling point of this compound are -936 and 761 respectively. The PH3 Lewis structure has 8 valence electrons. Answered expert verified Write a Lewis structure for the phosphorus trifluoride molecule PF3.
PF 3 C 2 F 4 SbH 3 20 Watch For unlimited access to Homework Help a Homework subscription is required. So if we place our pian center and its attached to three field that means its already taken 123 And it has 11 pair. Find out the total number of valence electrons in PF3 which is 26.
Include all lone pairs of electrons. Add your answer and earn points. Include all lone pair electrons.
Get 14 pages draw the lewis structure for pf3 analysis in PDF format. PF3 Draw the Lewis structure for PF3 in the box at the right inclĭraw the Lewis structure for PF3 in the box at the right including lone pairsīent Tetrahedral Linear Trigonal planar Trigonal pyramidalĭraw the Lewis structure of SF2 showing all lone pairs. Lewis Structure of ICl3 for counting valence electrons around the terminal chlorine atoms.
Steric number of PF3 3 1 4. It is a toxic compound but is used in several industries. Well put the P at the center and weve got three Fs.
It is six in total where three valence electrons are needed by the phosphorus atom and one each by three fluorine atoms. Determine which statement best describes the Lewis acid or base properties of the compounds. It has sp3 Hybridization and the bond angle is approximately 1095.
Phosphorus 5 valence electrons. 180 degree 120 degree 1095 degree. A step-by-step explanation of how to draw the PF3 Lewis Dot Structure Phosphorous trifluorideFor the PF3 structure use the periodic table to find the tota.
This liquid can be colorless as well. First week only 499. The molecule is trigonal pyramidal-shaped and is.
Draw the Lewis structure for BF_3 in the box at the right including lone pairs. To sketch the ICl3 Lewis structure by following these instructions. Linear Bent Trigonal planar Trigonal pyramidal Tetrahedral What is the F-B-F bond angle.
Advertisement Pipelock560 is waiting for your help. 1 answer 0 watching 816 views 10 Nov 2019 draw a lewis structure for each of the following compoundsInclude all lone pair electrons. Include all lone pair electrons.
Trigonal planar O bent O square pyramidal Linear trigonal bipyramidal T-shaped see-saw Question. C2F4 draw structure. Nelly Stracke Lv2 11 Aug 2019 Unlock all answers Get 1 free homework help answer.
Heres how you can draw the PF 3 lewis structure step by step. Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. The sp orbital with the lone pair on Plg is larger than on PF3.
Draw the molecule by placing atoms on the grid and connecting them with bonds. Okay so I have to draw the little structures of PCL three and pc all five. And then around the.
On the periodic table. The molar mass of this compound is 13733 gmol. Draw a Lewis structure for each of the following compounds.
PF3 and Pl3 both have phosphorus sp2 hybridized with a lone pair in. Steric number of PF3 Number of bonded atoms attached to phosphorous Lone pair on phosphorous As per the lewis structure of PF3 the central phosphorous atom is bonded with three fluorine atoms and it contains one lone pair also. PF3 draw structure.
Well start by putting electrons between each atom two of them to form a chemical bond. ICl3 Lewis dot Structure by counting valence electrons on the iodine atom. Plus Fluorine we have 7 but we have three Fluorines so we5 plus 21.
Solution for Draw the Lewis structure for formaldehyde CH2O. Now there can be questions about the. Draw the Lewis structure of PF3 and Plg.
Both can act as Lewis bases. Lets put them like this. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.
Lewis dot Structure for ICl3 generated from step-1 and step-2. PF3 draw structure. O PF3 and Plg both have phosphorus sp hybridized with a lone pair in an sp orbital.
PF3 Draw the Lewis structure. Find out the number of valence electrons further needed of a single PF3 molecule to stabilize itself. Include all hydrogens and lone pairs explicitly.
He had a five billion until has seven Valence Electron. If you can do those Lewis structures PF 3 will be easy. It is a volatile liquid that reacts with water and releases HCl gas.
Below are the steps to draw the lewis structure of the PF3 molecule 1. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms having a chemical formula of PCl3.

Chemistry Learning Made Easy Pf3 Lewis Structure And Molecular Geometry Youtube

Solved Draw The Lewis Dot Structure For Ch Cl Determine The Chegg Com

How To Draw Pf3 Lewis Structure Science Education And Tutorials

Pf3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Pf3 Lewis Structure Phosphorus Trifluoride Youtube

Pf3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
0 comments
Post a Comment